Energy is the power to do work. The power of thermal energy takes the form of heat.
It is important to differentiate between heat and temperature. A thermometer measures temperature, not heat. Heat can be defined as the energy in the random movement of atoms and molecules within a substance. The faster the atoms and molecules vibrate, the greater the heat, or thermal energy. Heat and temperature are of course closely linked. Increasing the heat of a substance – in other words, applying energy to cause its atoms and molecules to vibrate faster – will probably result in an increase in its temperature. But when, for instance, water has reached boiling point, increasing its thermal energy will not raise its temperature any further; instead, it will convert the water into steam.
Contents
Origins and Transfer of Thermal Energy
The primary source of heat is the sun, whose core is a mass of fast-moving gaseous particles. Radiant heat from the sun provides us with a powerful, though not continuous, stream of solar thermal energy. Other types of energy can also be converted into thermal energy; friction, for instance, is a way of converting mechanical energy into thermal energy. When we burn fossil fuels, we are using combustion to convert stored solar energy into thermal energy. Thermal energy heats our houses, powers steam-driven processes, and enables us to generate electricity.
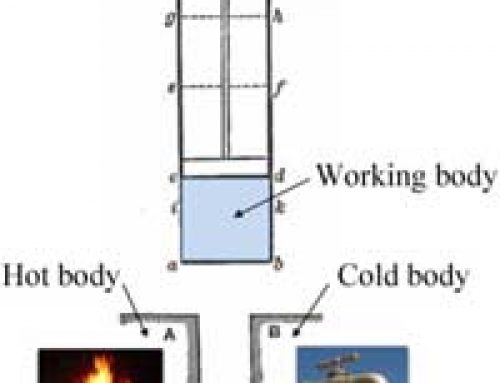
Applications of thermal energy involve the transference of heat from a hot substance to a colder one, in order to increase the temperature or bring about a change of state, from solid to liquid or liquid to gas. Heat transference happens through one of three different methods, or by a combination of these methods.
Conduction
Thermal energy can be transferred through a solid substance, or directly from one substance to another, by conduction. In conduction, heat is passed on from one molecule to the next. Because molecules vibrate more when hot, they collide more frequently with other molecules, causing those to vibrate more and become hot in turn. It is the heat, not the molecules themselves, that travels through the substance. Whether or not a particular substance is a good conductor depends upon its molecular composition.
Metals are generally good conductors; for instance, if one side of a block of aluminium is heated, the heat very quickly travels through to the other side. Other substances barely conduct heat at all, and these can be used as insulators – for example, lagging hot water tanks to prevent heat loss. Gases and liquids are not good conductors, but they do transfer thermal energy by convection.
Convection
In convection, thermal energy is transferred by the movement of hot particles. Convection takes place in gases and liquids but not in solids, whose particles cannot move freely. We all know that ‘hot air rises’, and this, in a nutshell, sums up convection. Cold air – or liquid – is denser than hot air or liquid. Therefore the hot particles move upwards, taking the place of the cold particles. Convected heat always moves towards a colder place and it is the hot particles, containing thermal energy, that travel. Convection heaters work by blowing out hot air particles, which then disperse into the colder air.
Radiation
Thermal energy is emitted by hot objects in the form of radiation. This is how the heat from the sun reaches Earth; radiated energy can travel across space because, unlike conduction and convection, this type of heat transference does not require any particles of matter. Radiant energy travels in electromagnetic waves. Within the electromagnetic spectrum, the different types of electromagnetic waves are divided into sections, according to wavelength.
At the bottom, with the longest wavelengths, are radio waves, followed by microwaves, infra-red waves, visible light waves including ultra-violet, X-rays and finally gamma rays which have the shortest wavelength. Thermal waves are within the infra-red waveband, and travel in just the same way as light waves. A simple application of radiant thermal energy is in infra-red lamps, which produce electrically-generated thermal heat that is transferred by radiation to the body, to ease muscular pain.
Making the Most of Thermal Energy
In daily life, a significant amount of thermal energy is wasted. Heat is produced during manufacturing processes and not used. Heat is lost from poorly-insulated buildings. although we try to minimise heat loss by installing better insulation and double glazing. Also, more of the heat left over from industrial processes is being recovered and used. More Combined Heat and Power plants are being built. And research is on-going into other potential methods of exploiting thermal energy. One such project is Ocean Thermal Energy Conservation, which is looking into the possibility of retrieving energy from the solar energy trapped in tropical oceans with a view to using this on a commercial scale in the future.